
What happens if you use a bottle with a narrower or wider neck-or a cylindrical drinking glass with no neck? Extra: Try the activity with different-shaped containers.What happens? How was the result different? Extra: Try the activity without the dish soap.Pour the yeast mixture into the bottle then quickly step back, and watch your reaction go! What happens? How long does the reaction last?.In a measuring cup mix together one tablespoon of yeast and three tablespoons of warm water.Let them drip down the inside of the bottle, but do not mix. If you want to give your foam stripes like some toothpastes, put the drops along the inside rim of the bottle’s mouth. If you want to make your foam a single color, add a few drops of food coloring directly into the hydrogen peroxide, and swirl the bottle gently to mix.Add a big squirt of dish soap into the bottle, and swirl gently to mix.Measure 1/2 cup of hydrogen peroxide, and carefully pour it into the bottle.Place your plastic bottle on the tray or tub so that it is easy to clean up all the foam. Gather your materials in the location where you plan to do your activity.(Note: although the product of this activity resembles toothpaste, it is not toothpaste, so do not attempt to use it!)
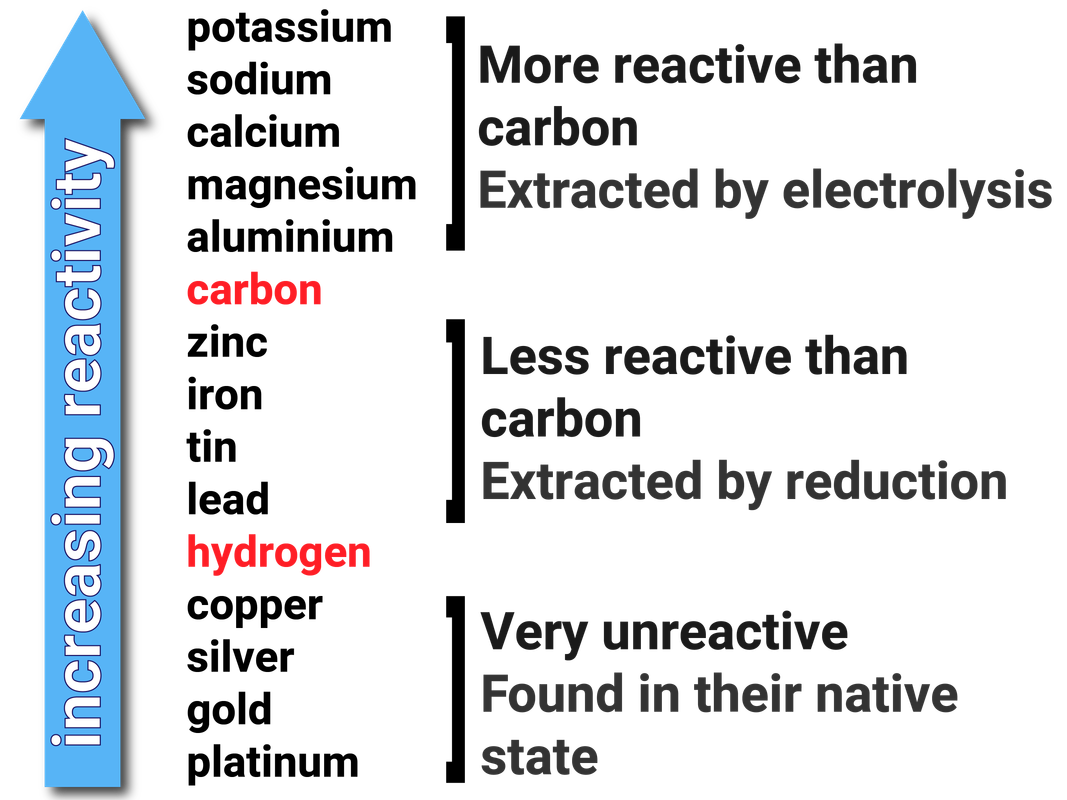 Put on your safety glasses to do this activity because hydrogen peroxide can irritate your eyes. Different-shaped bottles or glasses (optional). Location for the activity that can tolerate spills (of hydrogen peroxide as well as possibly food coloring), such as a kitchen or bathroom-or an outdoor location. Dry yeast (found in the baking section of the grocery store). This foam looks like a giant squeeze of toothpaste-almost big enough for an elephant! But adding a little dish soap provides additional surface tension, allowing the bubbles to get trapped and creating lots of foam. These bubbles would usually escape from the liquid and pop quickly. This means that if you mix yeast with hydrogen peroxide, the hydrogen peroxide will rapidly break down into water and oxygen gas. Catalase is present in almost all living things that are exposed to oxygen, and it helps them break down naturally occurring hydrogen peroxide. Yeast is an organism that contains a special chemical called catalase that can act as a catalyst to help break down hydrogen peroxide. But you can make that reaction happen faster! How? By adding a catalyst. Normally this breakdown happens very slowly. When hydrogen peroxide breaks down, it turns into oxygen (O 2) and water (H 2O). It also breaks down when exposed to light, which is why it usually comes in dark brown bottles. You usually find it in a 3 percent concentration (although higher concentrations are available, they are more dangerous and must be handled carefully). It is available in different strengths, or concentrations. But what is it? It is a liquid made from hydrogen atoms and oxygen atoms (its chemical formula is H 2O 2).
Put on your safety glasses to do this activity because hydrogen peroxide can irritate your eyes. Different-shaped bottles or glasses (optional). Location for the activity that can tolerate spills (of hydrogen peroxide as well as possibly food coloring), such as a kitchen or bathroom-or an outdoor location. Dry yeast (found in the baking section of the grocery store). This foam looks like a giant squeeze of toothpaste-almost big enough for an elephant! But adding a little dish soap provides additional surface tension, allowing the bubbles to get trapped and creating lots of foam. These bubbles would usually escape from the liquid and pop quickly. This means that if you mix yeast with hydrogen peroxide, the hydrogen peroxide will rapidly break down into water and oxygen gas. Catalase is present in almost all living things that are exposed to oxygen, and it helps them break down naturally occurring hydrogen peroxide. Yeast is an organism that contains a special chemical called catalase that can act as a catalyst to help break down hydrogen peroxide. But you can make that reaction happen faster! How? By adding a catalyst. Normally this breakdown happens very slowly. When hydrogen peroxide breaks down, it turns into oxygen (O 2) and water (H 2O). It also breaks down when exposed to light, which is why it usually comes in dark brown bottles. You usually find it in a 3 percent concentration (although higher concentrations are available, they are more dangerous and must be handled carefully). It is available in different strengths, or concentrations. But what is it? It is a liquid made from hydrogen atoms and oxygen atoms (its chemical formula is H 2O 2). 
You might be familiar with hydrogen peroxide as an antiseptic used to clean cuts and scrapes, which it does by killing bacteria.
With just a few ingredients you can make something that looks like foamy toothpaste being squeezed from a tube-but so big that it looks almost fit for an elephant! Create a giant foaming reaction, and use science to wow your friends with this classic activity.







 0 kommentar(er)
0 kommentar(er)
